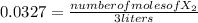Chemistry, 23.07.2020 21:01 trevorhenyan51
For the reaction system, H2(g) + X2(g) <--> 2HX(g), Kc = 24.4 at 300 K. A system made up from these components which is at equilibrium contains 0.150 moles of H2 and 0.600 moles of HX in a 3.00 liter container. Calculate the number of moles of X2(g) present at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2



Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
For the reaction system, H2(g) + X2(g) <--> 2HX(g), Kc = 24.4 at 300 K. A system made up from...
Questions

Mathematics, 25.11.2019 00:31



Mathematics, 25.11.2019 00:31

English, 25.11.2019 00:31

Mathematics, 25.11.2019 00:31

Spanish, 25.11.2019 00:31



Mathematics, 25.11.2019 00:31

Chemistry, 25.11.2019 00:31





Chemistry, 25.11.2019 00:31




Mathematics, 25.11.2019 00:31

![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0711/9653/e45ae.png)
![Kc=\frac{[HX]^{2} }{[H_{2} ]*[X_{2} ]}](/tpl/images/0711/9653/32146.png)
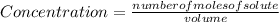 :
:![[H_{2} ]=\frac{0.150 moles}{3 liters}=0.05 \frac{moles}{liter}](/tpl/images/0711/9653/dc861.png)
![[HX]=\frac{0.600 moles}{3 liters}=0.2 \frac{moles}{liter}](/tpl/images/0711/9653/1aa32.png)
![24.4=\frac{0.2^{2} }{0.05*[X_{2} ]}](/tpl/images/0711/9653/346dd.png)
![[X_{2} ]=\frac{0.2^{2} }{0.05*24.4}](/tpl/images/0711/9653/ff5dd.png)
![[X_{2} ]=\frac{number of moles of X_{2} }{volume}](/tpl/images/0711/9653/ba7b5.png) , and the volume being 3 liters:
, and the volume being 3 liters: