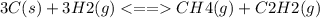Chemistry, 23.07.2020 06:01 quintencoffman2
Consider the following system, which is at equilibrium, 3C(s) + 3H2(g) <--> CH4(g) + C2H2(g) The result of removing some C(s) from the system will be:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

You know the right answer?
Consider the following system, which is at equilibrium, 3C(s) + 3H2(g) <--> CH4(g) + C2H2(g) T...
Questions


Biology, 24.06.2019 10:30




Mathematics, 24.06.2019 10:30








Mathematics, 24.06.2019 10:30

English, 24.06.2019 10:30

Mathematics, 24.06.2019 10:30

Mathematics, 24.06.2019 10:30

French, 24.06.2019 10:30