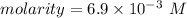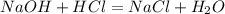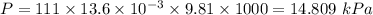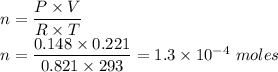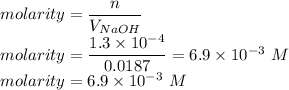Chemistry, 24.07.2020 17:01 taylorclarkx17
The volume of a sample of pure HCl gas was 221 mL at 20°C and 111 mmHg. It was completely dissolved in about 50 mL of water and titrated with an NaOH solution; 18.7 mL of the NaOH solution was required to neutralize the HCl. Calculate the molarity of the NaOH solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1


Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
The volume of a sample of pure HCl gas was 221 mL at 20°C and 111 mmHg. It was completely dissolved...
Questions

Mathematics, 25.03.2021 06:40

History, 25.03.2021 06:40






Mathematics, 25.03.2021 06:40

Mathematics, 25.03.2021 06:40

Mathematics, 25.03.2021 06:40

History, 25.03.2021 06:40

Mathematics, 25.03.2021 06:40


Mathematics, 25.03.2021 06:40


Chemistry, 25.03.2021 06:40


Chemistry, 25.03.2021 06:40

Biology, 25.03.2021 06:40

Mathematics, 25.03.2021 06:40