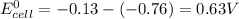Chemistry, 24.07.2020 22:01 bellagracebulle8018
Pb2+(aq) + 2 e- --> Pb(s) Eo = -0.13V Zn2+(aq) + 2 e- --> Zn(s) Eo = -0.76V 9. Given the half-cell potentials above, when the reaction Zn(s) + Pb2+(aq) --> Zn2+(aq) + Pb(s) is made into a voltaic cell, the Ecell is: A. 0.63 V B. -0.63 V C. 0.89 D. 0.89

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Pb2+(aq) + 2 e- --> Pb(s) Eo = -0.13V Zn2+(aq) + 2 e- --> Zn(s) Eo = -0.76V 9. Given the half-...
Questions

Mathematics, 13.07.2019 07:20

Social Studies, 13.07.2019 07:20

Mathematics, 13.07.2019 07:20

Advanced Placement (AP), 13.07.2019 07:20


Mathematics, 13.07.2019 07:20

English, 13.07.2019 07:20




Health, 13.07.2019 07:20

Mathematics, 13.07.2019 07:20

Mathematics, 13.07.2019 07:20

Mathematics, 13.07.2019 07:20

Mathematics, 13.07.2019 07:20

Social Studies, 13.07.2019 07:20



English, 13.07.2019 07:20

Mathematics, 13.07.2019 07:20

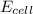 is 0.63 V
is 0.63 V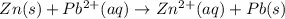
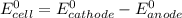
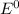 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Pb^{2+}/Pb]}= -0.13V](/tpl/images/0712/6436/bacf1.png)
![E^0_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0712/6436/4cd18.png)
![E^0_{cell}=E^0_{[Pb^{2+}/Pb]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0712/6436/22253.png)