
Chemistry, 25.07.2020 03:01 jessica112776
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(1)= -174.5kJ/mol. I have no clue how to start this question and need help including the formulas so I know how to do it and some step by step commentary.


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(...
Questions



Mathematics, 01.02.2022 07:20


Mathematics, 01.02.2022 07:20

English, 01.02.2022 07:20




English, 01.02.2022 07:30


Mathematics, 01.02.2022 07:30


Mathematics, 01.02.2022 07:30


History, 01.02.2022 07:30

Physics, 01.02.2022 07:30


Computers and Technology, 01.02.2022 07:30

Mathematics, 01.02.2022 07:30

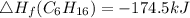
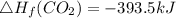
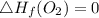
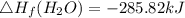
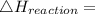 8 x - 393.5 - 8 x 285.82 + 174.5x 1
8 x - 393.5 - 8 x 285.82 + 174.5x 1 

