

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

You know the right answer?
Consider the reaction 2N2(g) O2(g)2N2O(g) Using the standard thermodynamic data in the tables linked...
Questions

Mathematics, 13.12.2019 09:31

Social Studies, 13.12.2019 09:31


History, 13.12.2019 09:31


Mathematics, 13.12.2019 09:31


Mathematics, 13.12.2019 09:31

Mathematics, 13.12.2019 09:31

History, 13.12.2019 09:31




Chemistry, 13.12.2019 09:31

Mathematics, 13.12.2019 09:31

Health, 13.12.2019 09:31

Physics, 13.12.2019 09:31

Chemistry, 13.12.2019 09:31


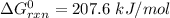
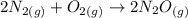
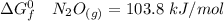
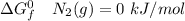
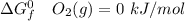
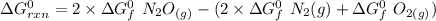
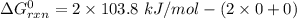
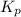 can be expressed as :
can be expressed as :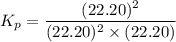
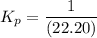
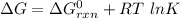
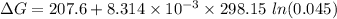
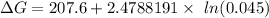

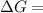 199.912952 kJ
199.912952 kJ


