
Chemistry, 27.07.2020 01:01 ayoismeisjuam
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazoic acid is 2.20×10−5. Use the method of successive approximations in your calculations or the quadratic formula.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazo...
Questions

Mathematics, 19.11.2020 09:50

Mathematics, 19.11.2020 09:50

Mathematics, 19.11.2020 09:50

Biology, 19.11.2020 09:50

Mathematics, 19.11.2020 09:50


Computers and Technology, 19.11.2020 09:50

Mathematics, 19.11.2020 09:50

Health, 19.11.2020 09:50


Computers and Technology, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00


Advanced Placement (AP), 19.11.2020 14:00

English, 19.11.2020 14:00


Mathematics, 19.11.2020 14:00

Chemistry, 19.11.2020 14:00


Mathematics, 19.11.2020 14:00

![[H^+]=0.000285](/tpl/images/0713/5320/5d625.png)
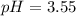
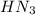 ). So:
). So:
![Ka=\frac{[H^+][N_3^-]}{[HN_3]}](/tpl/images/0713/5320/574a9.png)
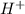 produced we will have 1 mol of
produced we will have 1 mol of 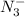 . So, we can use "X" for the unknown values and replace in the Ka equation:
. So, we can use "X" for the unknown values and replace in the Ka equation:![Ka=\frac{X*X}{[HN_3]}](/tpl/images/0713/5320/c6a9a.png)
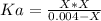
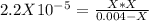
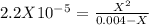
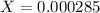
![pH=-Log[H^+]=-Log[0.000285]=3.55](/tpl/images/0713/5320/8cdec.png)


