

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
The equilibrium constants for the chemical reaction N 2(g) + O 2(g) 2NO(g) are K P = 1.1 × 10 –3 and...
Questions

English, 26.06.2021 15:50



Mathematics, 26.06.2021 15:50

Mathematics, 26.06.2021 15:50

Business, 26.06.2021 15:50


History, 26.06.2021 15:50



History, 26.06.2021 15:50

Biology, 26.06.2021 16:00

Spanish, 26.06.2021 16:00

English, 26.06.2021 16:00

English, 26.06.2021 16:00

Social Studies, 26.06.2021 16:00

History, 26.06.2021 16:00

English, 26.06.2021 16:00


English, 26.06.2021 16:00

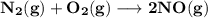
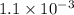
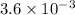
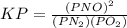
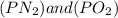 is defined in the denominator section and the value of
is defined in the denominator section and the value of 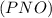 is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).
is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).

