
Chemistry, 27.07.2020 01:01 tammydbrooks43
1. What is the net ionic equation for the reaction that occurs when aqueous solutions of AgNO3 and CaCl2 are mixed and a precipitate forms? A. Ca+2(aq) + NO3-(aq) Ca(NO3)2(aq) B. Ag2+(aq) + 2Cl-(aq) AgCl2(s) C. Cl−(aq) + Ag+(aq) ⟶ AgCl(s) D. None of the above because no reaction occurs

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1


Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
1. What is the net ionic equation for the reaction that occurs when aqueous solutions of AgNO3 and C...
Questions

History, 16.04.2021 07:50

Chemistry, 16.04.2021 07:50


History, 16.04.2021 07:50

Mathematics, 16.04.2021 07:50

Chemistry, 16.04.2021 07:50




Mathematics, 16.04.2021 07:50



Mathematics, 16.04.2021 07:50






Business, 16.04.2021 07:50

Biology, 16.04.2021 07:50




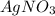 are:
are: and
and 
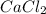 are:
are: and
and 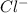
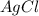 and
and 
 and an aqueous state for
and an aqueous state for 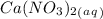 .
. 

