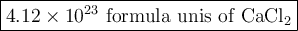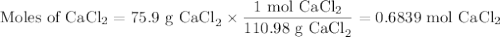Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3


Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
How many molecules of CaCl2 are equivalent to 75.9g CaCl2 (Ca=40.08g/mol, CL=35.45g/mol)...
Questions




Mathematics, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01



History, 21.10.2020 21:01




Advanced Placement (AP), 21.10.2020 21:01



History, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01