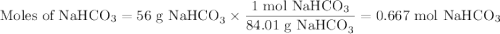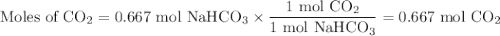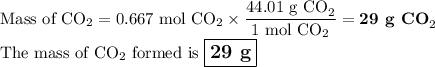Chemistry, 27.07.2020 07:01 fdougie111
En la reacción química. NaHCO3 + HCl → CO2 + NaCl + H2O Cuando reaccionan 56 g de NaHCO3 los g de CO2 liberados son: Nota: Solo escribir el valor numérico, sin unidades y dos lugares después del punto.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
En la reacción química. NaHCO3 + HCl → CO2 + NaCl + H2O Cuando reaccionan 56 g de NaHCO3 los g de CO...
Questions

Mathematics, 23.04.2020 23:58




Social Studies, 23.04.2020 23:58


Advanced Placement (AP), 23.04.2020 23:58


Social Studies, 23.04.2020 23:58

Mathematics, 23.04.2020 23:59

Mathematics, 23.04.2020 23:59






Business, 23.04.2020 23:59



Mathematics, 23.04.2020 23:59