
Consider the half reaction below.
Which statement best describes what is taking place?
Chlorine is losing electrons and being oxidized. Chlorine is losing electrons and being reduced. Chlorine is gaining electrons and being oxidized. Chlorine is gaining electrons and being reduced.
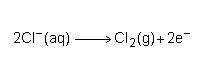

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
Consider the half reaction below.
Which statement best describes what is taking place?
...
...
Questions


Mathematics, 03.04.2020 00:30




Mathematics, 03.04.2020 00:30

Mathematics, 03.04.2020 00:30

Mathematics, 03.04.2020 00:30

Geography, 03.04.2020 00:30

Mathematics, 03.04.2020 00:30

Mathematics, 03.04.2020 00:30

Mathematics, 03.04.2020 00:30

Mathematics, 03.04.2020 00:30

Mathematics, 03.04.2020 00:30

Chemistry, 03.04.2020 00:30



Mathematics, 03.04.2020 00:30


English, 03.04.2020 00:30



