
Chemistry, 28.07.2020 05:01 FavvBella84
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of methane, CHA?
Select one:
O a. 19.26
O b. 38.52
O c. 15.0
O d. 24.7

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of met...
Questions

Mathematics, 16.03.2020 18:37

Computers and Technology, 16.03.2020 18:37


Mathematics, 16.03.2020 18:37

Social Studies, 16.03.2020 18:37


Spanish, 16.03.2020 18:37


History, 16.03.2020 18:37





Mathematics, 16.03.2020 18:37

Social Studies, 16.03.2020 18:37


Mathematics, 16.03.2020 18:37

Chemistry, 16.03.2020 18:37


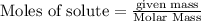
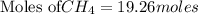
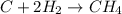
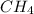 is produced by = 2 moles of
is produced by = 2 moles of 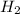
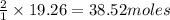 of
of 

