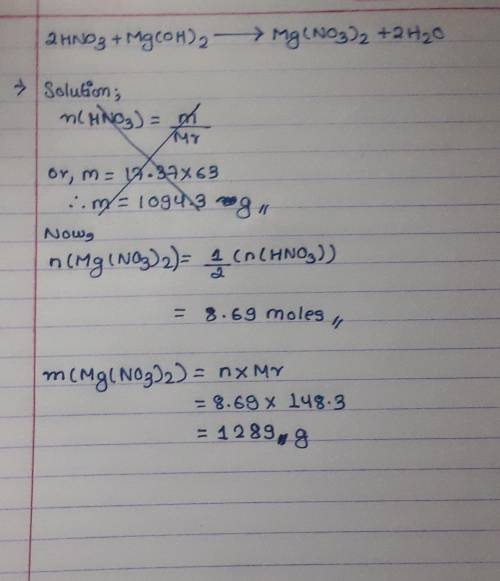Chemistry, 27.07.2020 05:01 dcarranza626
For the reaction 2HNO3 + Mg(OH)2 + Mg(NO3)2 + 2H20, how many grams of magnesium
nitrate are produced from 17.37 mol of nitric acid, HNO3?
Select one:
O a. 1290
Ob. 859
O c. 5160
Od. 1080

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
For the reaction 2HNO3 + Mg(OH)2 + Mg(NO3)2 + 2H20, how many grams of magnesium
nitrate are produce...
Questions




Mathematics, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00


Chemistry, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00