
Chemistry, 29.07.2020 05:01 graycelynn123
A mixture of water and graphite is heated to 600 K in a 1 L container. When the system comes to equilibrium it contains 0.17 mol of H2, 0.17 mol of CO, 0.74 mol of H2O, and some graphite. Some O2 is added to the system and a spark is applied so that the H2 reacts completely with the O2.
Find the amount of CO in the flask when the system returns to equilibrium.
Express your answer to two significant figures and include the appropriate units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

Chemistry, 23.06.2019 12:20
Amatch has about 21 milligrams of red phosphorus coating the tip. how many atoms of phosphorus is this?
Answers: 1
You know the right answer?
A mixture of water and graphite is heated to 600 K in a 1 L container. When the system comes to equi...
Questions

Mathematics, 29.06.2020 04:01




Mathematics, 29.06.2020 05:01


Mathematics, 29.06.2020 05:01

Mathematics, 29.06.2020 05:01


Health, 29.06.2020 05:01


Mathematics, 29.06.2020 05:01

Arts, 29.06.2020 05:01

Mathematics, 29.06.2020 05:01

Physics, 29.06.2020 05:01

Mathematics, 29.06.2020 05:01



English, 29.06.2020 05:01

Mathematics, 29.06.2020 05:01

![K_c= \dfrac{[CO][H_2]}{[H_2O]}](/tpl/images/0714/6363/51350.png)
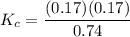
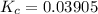
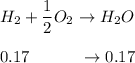
![0.03905 = \dfrac{[0.17+x][x]}{[0.91 -x]}](/tpl/images/0714/6363/fa6d0.png)


