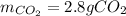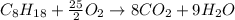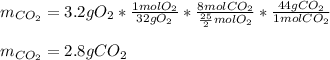A major component of gasoline is octane, C8H18. When octane is burned in air, it chemically reacts with oxygen gas (O2) to produce carbon dioxide (CO2) and water (H2O) . What mass of carbon dioxide is produced by the reaction of 3.2g of oxygen gas? Round your answer to 2 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
A major component of gasoline is octane, C8H18. When octane is burned in air, it chemically reacts w...
Questions

English, 21.01.2022 02:00


Chemistry, 21.01.2022 02:00


English, 21.01.2022 02:00


English, 21.01.2022 02:00

Mathematics, 21.01.2022 02:00

Mathematics, 21.01.2022 02:00



English, 21.01.2022 02:00

Mathematics, 21.01.2022 02:00


Mathematics, 21.01.2022 02:00

History, 21.01.2022 02:00


Mathematics, 21.01.2022 02:00