
Chemistry, 29.07.2020 21:01 hubbabubba0715
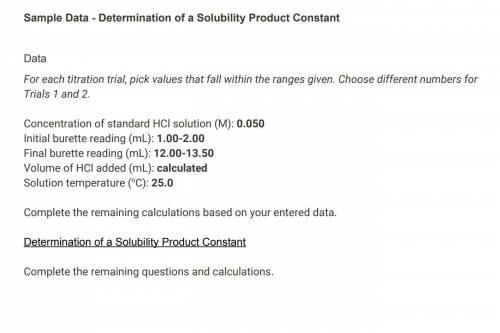
Concentration of standard HCl solution (M) 0.050 Saved Table view List view Trial 1 Trial 2 Initial burette reading (mL) 1.00 2.00 Final burette reading (mL) 12.00 13.50 Volume of HCl added (mL) 13.00 15.50 Solution temperature (°C) 25.0 25.0 (1pts) Average volume HCl added (mL) 14.25 Saved (2pts) Concentration of OH− (M) (2pts) Concentration of Ca2+ (M) (2pts) Value of Ksp for Ca(OH)2

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

You know the right answer?
Concentration of standard HCl solution (M) 0.050 Saved Table view List view Trial 1 Trial 2 Initial...
Questions

Mathematics, 04.04.2020 03:25

Mathematics, 04.04.2020 03:25


Mathematics, 04.04.2020 03:25



Mathematics, 04.04.2020 03:25


English, 04.04.2020 03:26

Chemistry, 04.04.2020 03:26



Mathematics, 04.04.2020 03:26






Computers and Technology, 04.04.2020 03:27



