
Chemistry, 30.07.2020 01:01 pablohc200021
What would be the voltage (Ecell) of a voltaic cell comprised of Cr (s)/Cr3+(aq) and Fe (s)/Fe2+(aq) if the concentrations of the ions in solution were [Cr3+] = 0.75 M and [Fe2+] = 0.25 M at 298K?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
What would be the voltage (Ecell) of a voltaic cell comprised of Cr (s)/Cr3+(aq) and Fe (s)/Fe2+(aq)...
Questions



Mathematics, 21.08.2019 00:20



Mathematics, 21.08.2019 00:20

Mathematics, 21.08.2019 00:20


Mathematics, 21.08.2019 00:30


Mathematics, 21.08.2019 00:30

Mathematics, 21.08.2019 00:30

Mathematics, 21.08.2019 00:30

Social Studies, 21.08.2019 00:30

Mathematics, 21.08.2019 00:30


History, 21.08.2019 00:30

Mathematics, 21.08.2019 00:30

Mathematics, 21.08.2019 00:30

History, 21.08.2019 00:30

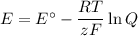
![Q = \dfrac{\text{[Fe}^{2+}]^{3}}{ \text{[Cr}^{3+}]^{2}} = \dfrac{0.25^{3}}{ 0.75^{2}} =\dfrac{0.0156}{0.562} = 0.0278\\\\E = 0.33 - \left (\dfrac{8.314 \times 298}{6 \times 96485}\right ) \ln(0.0278)\\\\=0.33 -0.00428 \times (-3.58) = 0.33 + 0.0153 = \textbf{0.35 V}\\\text{The cell potential is }\large\boxed{\textbf{0.35 V}}](/tpl/images/0715/1287/2ffcc.png)


