Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
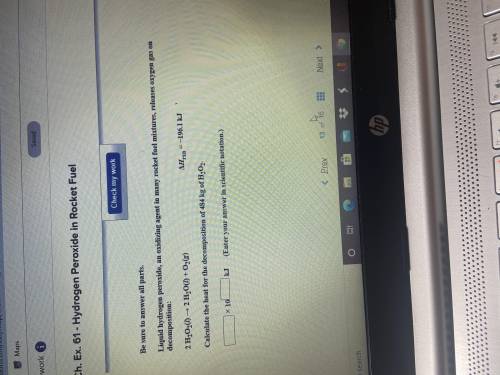
Liquid hydrogen peroxide an oxidizing agent in many rocket fuel mixtures, releases oxygen gas on dec...
Questions



Mathematics, 29.10.2020 21:20

Mathematics, 29.10.2020 21:20

Mathematics, 29.10.2020 21:20

Mathematics, 29.10.2020 21:20

English, 29.10.2020 21:20

Mathematics, 29.10.2020 21:20


Mathematics, 29.10.2020 21:20




English, 29.10.2020 21:20



Mathematics, 29.10.2020 21:20

Mathematics, 29.10.2020 21:20


Mathematics, 29.10.2020 21:20