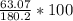Chemistry, 30.07.2020 03:01 christinafish9303
Aspirin (C9H8O4) is produced by the reaction of salicylic acid (C7H6O3, Molar mass = 138.1 g/mol) and acetic anhydride (C4H6O3, Molar mass = 102.1 g/mol) based on the BALANCED equation : C7H6O3(s) + C4H6O3(l ) → C9H8O4(s) + C2H4O2( l) If 63.07 grams of aspirin (Molar mass = 180.2 g/mol) was collected from an experiment when 138.1 grams C7H6O3 reacted with excess C4H6O3, what was the percent yield?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 11:10
Why would a doctor most likely restrict a patient's contact with other people while the patient receives internal radiation
Answers: 1
You know the right answer?
Aspirin (C9H8O4) is produced by the reaction of salicylic acid (C7H6O3, Molar mass = 138.1 g/mol) an...
Questions


Mathematics, 03.12.2020 02:00



Mathematics, 03.12.2020 02:00

Spanish, 03.12.2020 02:00







SAT, 03.12.2020 02:00


Chemistry, 03.12.2020 02:00


Social Studies, 03.12.2020 02:00

Mathematics, 03.12.2020 02:00


Arts, 03.12.2020 02:00

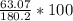 hould be used up in the reaction, yielding 180.2 g of C9H8O4. ⇒ Theoretical yield = 180.2g
hould be used up in the reaction, yielding 180.2 g of C9H8O4. ⇒ Theoretical yield = 180.2g