Chemistry, 30.07.2020 04:01 jadkins842
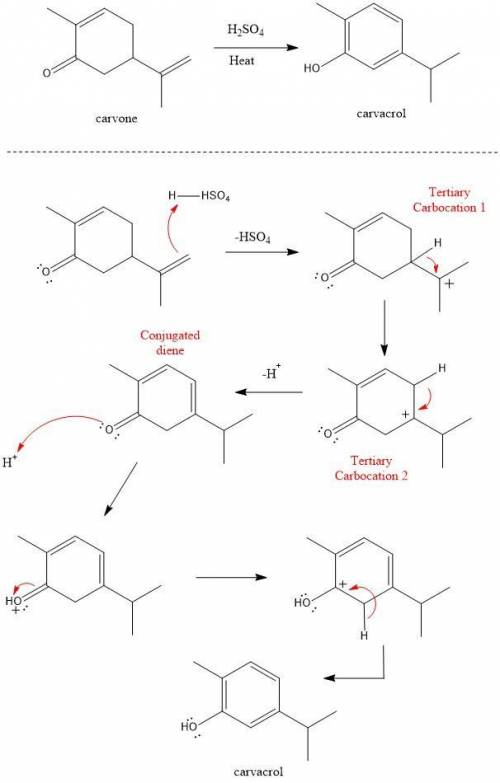
Heating carvone with aqueous sulfuric acid converts it into carvacrol. The mechanism involves the following steps:
1. The terminal alkene of carvone reacts with acid to form tertiary carbocation 1;
2. A hydride shift results in the formation of tertiary carbocation 2;
3. Deprotonation of the ring leads to conjugated diene 3;
4. Deprotonation at the α carbon leads to the product carvacrol.
Required:
Draw the mechanism and then draw the structure of tertiary carbocation 2.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Heating carvone with aqueous sulfuric acid converts it into carvacrol. The mechanism involves the fo...
Questions

Mathematics, 22.05.2021 18:30




Mathematics, 22.05.2021 18:30

Physics, 22.05.2021 18:30


Chemistry, 22.05.2021 18:30


English, 22.05.2021 18:30


Mathematics, 22.05.2021 18:30



History, 22.05.2021 18:30

Mathematics, 22.05.2021 18:30


Mathematics, 22.05.2021 18:30

Mathematics, 22.05.2021 18:30