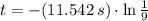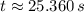Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2


Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
A compound decomposes with a half-life of 8.0 s and the half-life is independent of the concentratio...
Questions








English, 25.01.2021 20:40



Mathematics, 25.01.2021 20:40









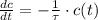
 - Time constant, measured in seconds.
- Time constant, measured in seconds.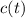 - Concentration of the compound as a function of time.
- Concentration of the compound as a function of time.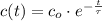
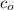 is the initial concentration of the compound.
is the initial concentration of the compound.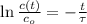
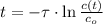
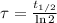
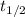 is the half-life of the composite decomposition, measured in seconds.
is the half-life of the composite decomposition, measured in seconds.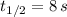 , then:
, then: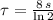
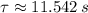
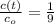 and
and