

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
Question 5 options: The cell potential for an electrochemical cell with a Zn, Zn2 half-cell and an A...
Questions

Mathematics, 16.11.2020 20:40



Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40



Advanced Placement (AP), 16.11.2020 20:40


English, 16.11.2020 20:40

History, 16.11.2020 20:40


Mathematics, 16.11.2020 20:40


Biology, 16.11.2020 20:40

English, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40



 ⇔
⇔  (reference study academy)
(reference study academy)
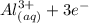 ⇔
⇔ 






