Chemistry, 30.07.2020 08:01 putaprincess16
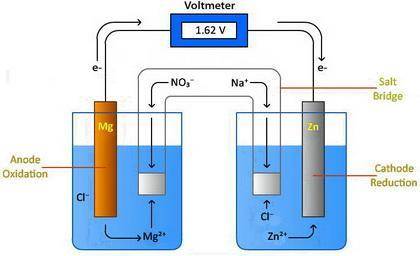
Design a voltaic cell using magnesium as one of the electrodes. Magnesium can be represented as either Metal A or Metal B in the above drawing. Use metal chlorides as the solutions in the two chambers. For example, magnesium chloride, (MgCl2) will be in solution in the chamber with the magnesium electrode. Use NaNO3 in the salt bridge. Select another element for the other electrode. Explain why you selected this element. Include information about the activity of the metal you select and the need for a spontaneous reaction. Metal A: Metal B: In the drawing, 1. Label the oxidation compartment: 2. Label the reduction compartment. 3. Label the direction of the flow of electrons. 4. Label the flow of the magnesium ions. 5. Label the flow of your selected element's ions. 6. What is leaving the salt bridge in the anode compartment? 7. What is leaving the salt bridge in the cathode compartment? 8. Write the oxidation and reduction half-reactions. 9. Calculate the chemical potential of your cell. Show all of your work.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
Design a voltaic cell using magnesium as one of the electrodes. Magnesium can be represented as eith...
Questions

Mathematics, 20.01.2021 01:00

Computers and Technology, 20.01.2021 01:00

Arts, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00




Social Studies, 20.01.2021 01:00

Biology, 20.01.2021 01:00

Biology, 20.01.2021 01:00



Mathematics, 20.01.2021 01:00

English, 20.01.2021 01:00


English, 20.01.2021 01:00