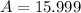In nature, oxygen has three common isotopes. The atomic masses and relative abundances of these isotopes are given in the table below. Isotope Atomic Mass (amu) Relative Abundance O-16 15.995 99.759% O-17 16.995 0.037% O-18 17.999 0.204% Calculate the average atomic mass of oxygen. Show all of your calculations below.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
In nature, oxygen has three common isotopes. The atomic masses and relative abundances of these isot...
Questions







English, 26.01.2020 22:31


Mathematics, 26.01.2020 22:31



Mathematics, 26.01.2020 22:31

World Languages, 26.01.2020 22:31

Mathematics, 26.01.2020 22:31



Mathematics, 26.01.2020 22:31


Health, 26.01.2020 22:31

History, 26.01.2020 22:31

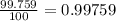
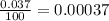


![A=\sum[(15.995\times 0.99759)+(16.995\times 0.00037)+(17.999 \times 0.00204)]](/tpl/images/0715/9581/687c5.png)