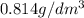Chemistry, 01.08.2020 01:01 koggebless
30cm^3 of a dilute solution of Ca(OH)2 required 11 cm^3 of 0.06 mol/dm^. Hcl for complete neutralization. Calculate the concentration of the Ca(OH)^2 solution in mol/dm^3 and g/dm^3

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
30cm^3 of a dilute solution of Ca(OH)2 required 11 cm^3 of 0.06 mol/dm^. Hcl for complete neutraliza...
Questions

Mathematics, 22.10.2021 20:50

Chemistry, 22.10.2021 20:50


Mathematics, 22.10.2021 20:50

English, 22.10.2021 20:50


Computers and Technology, 22.10.2021 20:50

Business, 22.10.2021 20:50

Mathematics, 22.10.2021 20:50



Mathematics, 22.10.2021 20:50

History, 22.10.2021 20:50




Mathematics, 22.10.2021 20:50


Mathematics, 22.10.2021 20:50

English, 22.10.2021 20:50

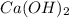 in
in 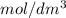 is 0.011 and in
is 0.011 and in 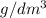 is 0.814
is 0.814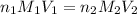
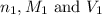 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 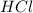
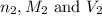 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 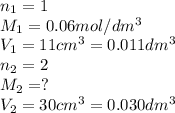
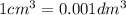
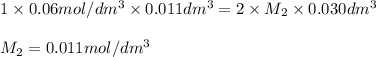
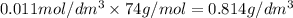
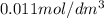 and
and