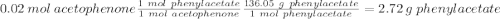Chemistry, 01.08.2020 02:01 bthakkar25
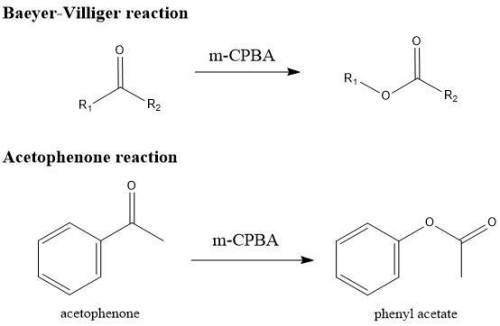
Bayer Villiger Provide a balanced chemical equation of the reaction performed in this experiment. Use structures and compound names to show ALL reactants and products involved. Baeyer-Villiger Reaction of Acetophenone Data Results
• Moles of acetophenone used: (Show calculations) 0.020 moles (2.40g/120.151 g mol-1 =0.0199 moles)
• Moles of mCPBA used: (Show calculations) 0.036 moles_(6.25 grams/ 172.56 g. mol-1)
• Expected mass of the product: (Show calculation. Clearly show the limiting and excess reactants)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Bayer Villiger Provide a balanced chemical equation of the reaction performed in this experiment. Us...
Questions

Social Studies, 12.09.2021 14:10

Law, 12.09.2021 14:10

English, 12.09.2021 14:10


Mathematics, 12.09.2021 14:10

Mathematics, 12.09.2021 14:10


Mathematics, 12.09.2021 14:10

Mathematics, 12.09.2021 14:20

Social Studies, 12.09.2021 14:20

History, 12.09.2021 14:20

Business, 12.09.2021 14:20

Physics, 12.09.2021 14:20

English, 12.09.2021 14:20


SAT, 12.09.2021 14:20


Mathematics, 12.09.2021 14:20


History, 12.09.2021 14:20