

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

You know the right answer?
A strontium hydroxide solution is prepared by dissolving 10.60 gg of Sr(OH)2Sr(OH)2 in water to make...
Questions

Chemistry, 14.10.2019 08:30

Mathematics, 14.10.2019 08:30

Mathematics, 14.10.2019 08:30


World Languages, 14.10.2019 08:30



Mathematics, 14.10.2019 08:30



English, 14.10.2019 08:30

History, 14.10.2019 08:30


Mathematics, 14.10.2019 08:30

Health, 14.10.2019 08:30



Mathematics, 14.10.2019 08:30


Mathematics, 14.10.2019 08:30

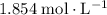 .
.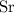 ,
,  , and
, and 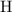 on a modern periodic table. Keep at least four significant figures in each of these atomic mass data.
on a modern periodic table. Keep at least four significant figures in each of these atomic mass data.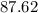 .
.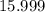 .
.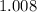 .
.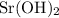 :
: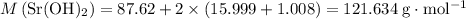 .
.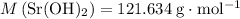 means that each mole of
means that each mole of 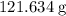 .
. 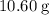 of
of 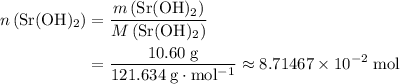 .
.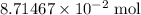 of
of 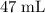 solution. Convert the unit of volume to liter:
solution. Convert the unit of volume to liter: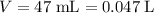 .
.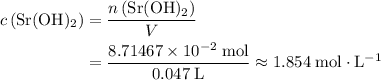 .
.

