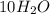Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
n-Butane (C4H10) is burned with stoichiometric amount of oxygen. Determine the mole fraction of carb...
Questions



Chemistry, 24.11.2020 07:00

Health, 24.11.2020 07:00


Mathematics, 24.11.2020 07:00

History, 24.11.2020 07:00

History, 24.11.2020 07:00

Mathematics, 24.11.2020 07:00

Mathematics, 24.11.2020 07:00


Mathematics, 24.11.2020 07:00

Mathematics, 24.11.2020 07:00

Biology, 24.11.2020 07:00

History, 24.11.2020 07:00


Spanish, 24.11.2020 07:00

Chemistry, 24.11.2020 07:00

Social Studies, 24.11.2020 07:00

English, 24.11.2020 07:00

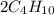 +
+ 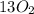 →
→ 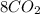 +
+