Chemistry, 01.08.2020 22:01 kaylijocombs
g If you have three identical containers (same volume) at the same temperature and pressure, each with a different gas. Container A has He, container B has Ne, and container C has O2. Which flask contains the largest number of molecules? Group of answer choices

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
g If you have three identical containers (same volume) at the same temperature and pressure, each wi...
Questions








Mathematics, 05.12.2021 03:00

English, 05.12.2021 03:00



Mathematics, 05.12.2021 03:00

Chemistry, 05.12.2021 03:00

Mathematics, 05.12.2021 03:00



Mathematics, 05.12.2021 03:00

Physics, 05.12.2021 03:00

English, 05.12.2021 03:00

Mathematics, 05.12.2021 03:00

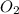 ) are all contained in separate identical containers with the same volume. And they are all stored at the same temperature, and pressure. Then, they'll all contain the same number of molecules. This is in line with Avogadro's law which states that "Equal volume of all gases, at the same temperature and pressure, have the same number of molecules."
) are all contained in separate identical containers with the same volume. And they are all stored at the same temperature, and pressure. Then, they'll all contain the same number of molecules. This is in line with Avogadro's law which states that "Equal volume of all gases, at the same temperature and pressure, have the same number of molecules."