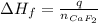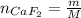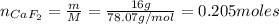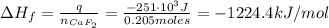Chemistry, 01.08.2020 23:01 kelsotay623
A 8.22-g sample of solid calcium reacted in excess fluorine gas to give a 16-g sample of pure solid CaF2. The heat given off in this reaction was 251 kJ at constant pressure. Given this information, what is the enthalpy of formation of CaF2(s)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3
You know the right answer?
A 8.22-g sample of solid calcium reacted in excess fluorine gas to give a 16-g sample of pure solid...
Questions




Chemistry, 19.02.2020 04:54

Arts, 19.02.2020 04:54





Mathematics, 19.02.2020 04:54




Computers and Technology, 19.02.2020 04:54






English, 19.02.2020 04:54