
Chemistry, 03.08.2020 14:01 zacharoo10
For the following equilibrium, Ag3PO4(s)↽−−⇀3Ag+(aq)+PO3−4(aq) If Ksp=2.4×10−28, what is the molar solubility of Ag3PO4? Report your answer in scientific notation with the correct number of significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

You know the right answer?
For the following equilibrium, Ag3PO4(s)↽−−⇀3Ag+(aq)+PO3−4(aq) If Ksp=2.4×10−28, what is the molar s...
Questions

Mathematics, 26.09.2021 22:20

Mathematics, 26.09.2021 22:20


Mathematics, 26.09.2021 22:20


Mathematics, 26.09.2021 22:20

Biology, 26.09.2021 22:20

Biology, 26.09.2021 22:20

Mathematics, 26.09.2021 22:20

English, 26.09.2021 22:20

Mathematics, 26.09.2021 22:20

English, 26.09.2021 22:20

History, 26.09.2021 22:20

Mathematics, 26.09.2021 22:20

Mathematics, 26.09.2021 22:20

Mathematics, 26.09.2021 22:20


English, 26.09.2021 22:20

Mathematics, 26.09.2021 22:20

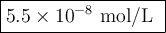
![K_{sp} =\text{[Ag$^{+}$]$^{3}$[PO$_{4}^{3-}$]} = (3x)^{3}x = 2.4 \times 10^{-28}\\27x^{4} = 2.4 \times 10^{-28}\\x^{4} = 8.89 \times 10^{-30}\\x = \sqrt[4]{8.89 \times 10^{-30}}\\= \mathbf{5.5\times 10^{-8}} \textbf{ mol/L}\\\text{The molar solubility of silver phosphate is $\large \boxed{\mathbf{5.5\times 10^{-8}}\textbf{ mol/L }}$}](/tpl/images/0716/8807/790d1.png)


