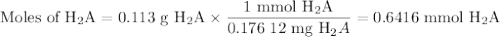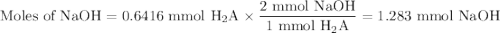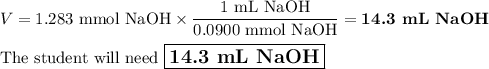Chemistry, 03.08.2020 14:01 lizzie3545
A chemistry student weighs out of ascorbic acid , a diprotic acid, into a volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with solution. Calculate the volume of solution the student will need to add to reach the final equivalence point. Round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
A chemistry student weighs out of ascorbic acid , a diprotic acid, into a volumetric flask and dilut...
Questions

Mathematics, 29.03.2022 04:10

History, 29.03.2022 04:10

Mathematics, 29.03.2022 04:20



Biology, 29.03.2022 04:30

Mathematics, 29.03.2022 04:30

Business, 29.03.2022 04:30

Mathematics, 29.03.2022 04:30





Biology, 29.03.2022 04:50



Computers and Technology, 29.03.2022 05:10

Physics, 29.03.2022 05:10