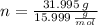Chemistry, 03.08.2020 14:01 ericwheeler821
An organic acid is composed of carbon (68.84%), hydrogen (4.96%), and oxygen (26.20%). Its molar mass is 122.12 g/mol. Determine the molecular formula of the compound.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
An organic acid is composed of carbon (68.84%), hydrogen (4.96%), and oxygen (26.20%). Its molar mas...
Questions


Mathematics, 29.07.2019 10:00

Health, 29.07.2019 10:00


Mathematics, 29.07.2019 10:00




Mathematics, 29.07.2019 10:00

History, 29.07.2019 10:00

Biology, 29.07.2019 10:00


Mathematics, 29.07.2019 10:00


Biology, 29.07.2019 10:00

Geography, 29.07.2019 10:00


History, 29.07.2019 10:00

Mathematics, 29.07.2019 10:00

History, 29.07.2019 10:00

 .
.
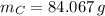
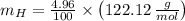
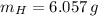
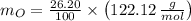

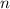 ), measured in moles, of each element are calculated by the following expression:
), measured in moles, of each element are calculated by the following expression: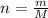
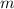 - Mass of the element, measured in grams.
- Mass of the element, measured in grams.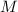 - Molar mass of the element, measured in grams per mol.
- Molar mass of the element, measured in grams per mol. )
)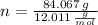

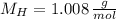 )
)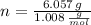
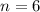
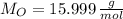 )
)