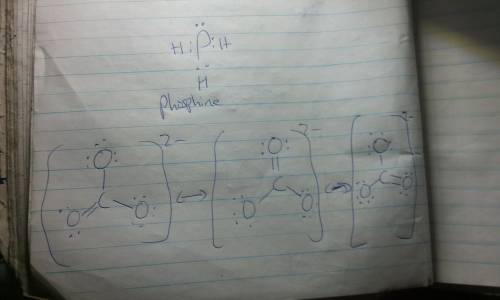
Resonance Structures are ways to represent the bonding in a molecule or ion when a single Lewis structure fails to describe accurately the actual electronic structure. Equivalent resonance structures occur when there are identical patterns of bonding within the molecule or ion. The actual structure is a composite, or resonance hybrid, of the equivalent contributing structures. Draw Lewis structures for thecarbonate ion and for phosphine in which the central atom obeys the octet rule. ... How many equivalent Lewis structures are necessary to describe the bonding in CO32-

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Resonance Structures are ways to represent the bonding in a molecule or ion when a single Lewis stru...
Questions

Arts, 14.01.2021 17:50


Social Studies, 14.01.2021 17:50



Mathematics, 14.01.2021 17:50

Mathematics, 14.01.2021 17:50

Mathematics, 14.01.2021 17:50

Mathematics, 14.01.2021 17:50

Engineering, 14.01.2021 17:50




Chemistry, 14.01.2021 17:50

English, 14.01.2021 17:50

Biology, 14.01.2021 17:50

Biology, 14.01.2021 17:50


Social Studies, 14.01.2021 17:50