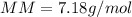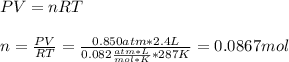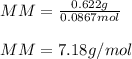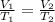Part 1. Determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 L at 287 K and 0.850 atm. Show your work. Part 2. If this sample was placed under extremely low temperature, describe how the actual volume would compare to the predicted volume. Explain your answer.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Part 1. Determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 L at 287 K and...
Questions




Mathematics, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20

Chemistry, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20

Biology, 19.03.2021 21:20

Computers and Technology, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20



Mathematics, 19.03.2021 21:20