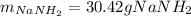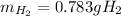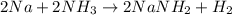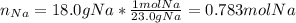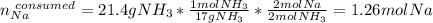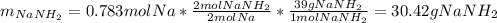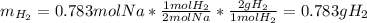Chemistry, 12.08.2020 05:01 mathwiznot45
Assuming that you start with 21.4 g of ammonia gas and 18.0 g of sodium metal and assuming that the reaction goes to completion, determine the mass (in grams) of each product.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2
You know the right answer?
Assuming that you start with 21.4 g of ammonia gas and 18.0 g of sodium metal and assuming that the...
Questions

Mathematics, 10.04.2021 01:00

Mathematics, 10.04.2021 01:00

Biology, 10.04.2021 01:00


Mathematics, 10.04.2021 01:00


Chemistry, 10.04.2021 01:00

Arts, 10.04.2021 01:00


Mathematics, 10.04.2021 01:00



Mathematics, 10.04.2021 01:00

English, 10.04.2021 01:00

Mathematics, 10.04.2021 01:00



Mathematics, 10.04.2021 01:00

Mathematics, 10.04.2021 01:00

English, 10.04.2021 01:00