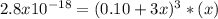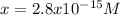Chemistry, 12.08.2020 06:01 shanilafaridor97hl
The solubility product for Ag3PO4 is 2.8 × 10‑18. What is the solubility of silver phosphate in a solution which also contains 0.10 moles of silver nitrate per liter?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

You know the right answer?
The solubility product for Ag3PO4 is 2.8 × 10‑18. What is the solubility of silver phosphate in a so...
Questions

Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Biology, 23.09.2020 14:01








Arts, 23.09.2020 14:01

Biology, 23.09.2020 14:01






Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

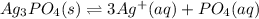
![Ksp=[Ag^+]^3[PO_4^-]](/tpl/images/0718/8186/a4d33.png)
 :
: