
Chemistry, 12.08.2020 06:01 murdocksfamilyouoxvm
Calculate the [H+] and pH of a 0.0010 M acetic acid solution. The Ka of acetic acid is 1.76×10−5. Use the method of successive approximations in your calculations.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3
You know the right answer?
Calculate the [H+] and pH of a 0.0010 M acetic acid solution. The Ka of acetic acid is 1.76×10−5. Us...
Questions

Mathematics, 04.03.2021 01:30

Advanced Placement (AP), 04.03.2021 01:30


Mathematics, 04.03.2021 01:30

Chemistry, 04.03.2021 01:30

Mathematics, 04.03.2021 01:30




Mathematics, 04.03.2021 01:30



Biology, 04.03.2021 01:30

Physics, 04.03.2021 01:30

Health, 04.03.2021 01:30

French, 04.03.2021 01:30


Advanced Placement (AP), 04.03.2021 01:30


![[H^+]=0.000123M](/tpl/images/0718/8547/72098.png)
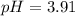
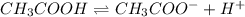
![Ka=\frac{[CH_3COO^-][H^+]}{[CH_3COOH]}](/tpl/images/0718/8547/15f82.png)
 could be written as:
could be written as:![1.74x10^{-5}=\frac{x*x}{[CH_3COOH]_0-x}=\frac{x*x}{0.0010M-x}](/tpl/images/0718/8547/89a17.png)
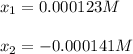
![pH=-log([H^+])=-log(0.000123)\\\\pH=3.91](/tpl/images/0718/8547/d511c.png)


