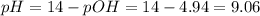Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Calculate the pH of a solution formed by mixing 250.0 mL of 0.15 M NH4Cl with 200.0 mL of 0.12 M NH3...
Questions

English, 08.10.2020 06:01

Mathematics, 08.10.2020 06:01

Mathematics, 08.10.2020 06:01

Mathematics, 08.10.2020 06:01



Chemistry, 08.10.2020 06:01

Mathematics, 08.10.2020 06:01

Mathematics, 08.10.2020 06:01

Mathematics, 08.10.2020 06:01



Mathematics, 08.10.2020 06:01

Biology, 08.10.2020 06:01

English, 08.10.2020 06:01

Mathematics, 08.10.2020 06:01


Computers and Technology, 08.10.2020 06:01


![[NH_{3}] = \frac{n_{NH_{3}}}{V_{T}} = \frac{C_{i}_{(NH_{3})}*Vi_{NH_{3}}}{V_{NH_{3}} + V_{NH_{4}^{+}}} = \frac{0.12 M*0.2 L}{0.2 L + 0.25 L} = 0.053 M](/tpl/images/0718/8632/272cb.png)
![[NH_{4}^{+}] = \frac{C_{i}_{(NH_{4}^{+})*V_{NH_{4}^{+}}}}{V_{NH_{3}} + V_{NH_{4}^{+}}} = \frac{0.15 M*0.25 L}{0.2 L + 0.25 L} = 0.083 M](/tpl/images/0718/8632/cd416.png)
![Kb = \frac{[NH_{4}^{+}][OH^{-}]}{[NH_{3}]}](/tpl/images/0718/8632/bdcae.png)
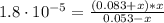

![pOH = -log([OH^{-}]) = -log(1.15 \cdot 10^{-5}) = 4.94](/tpl/images/0718/8632/c67ec.png)