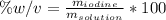Chemistry, 12.08.2020 05:01 Delgadojacky0206
Describe how you would prepare 500ml of 40% (w/v) aqueous iodine solution. [Atomic mass of iodine =127g/mol].

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1


Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1
You know the right answer?
Describe how you would prepare 500ml of 40% (w/v) aqueous iodine solution.
[Atomic mass of iodine =...
Questions


Chemistry, 04.12.2020 09:20

Health, 04.12.2020 09:20

Mathematics, 04.12.2020 09:20



Chemistry, 04.12.2020 09:20


English, 04.12.2020 09:20


Mathematics, 04.12.2020 09:20

Mathematics, 04.12.2020 09:20


Chemistry, 04.12.2020 09:20

Mathematics, 04.12.2020 09:20

Mathematics, 04.12.2020 09:20

Mathematics, 04.12.2020 09:20

Social Studies, 04.12.2020 09:20

Mathematics, 04.12.2020 09:20