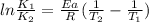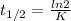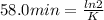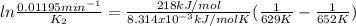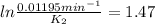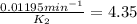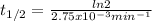Chemistry, 12.08.2020 05:01 alexmoy45p8yd7v
The decomposition of ethylene oxide(CH₂)₂O(g) → CH₄(g) + CO(g)is a first order reaction with a half-life of 58.0 min at 652 K. The activation energy of the reaction is 218 kJ/mol. Calculate the half-life at 629 K.

Answers: 1
Another question on Chemistry




Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
The decomposition of ethylene oxide(CH₂)₂O(g) → CH₄(g) + CO(g)is a first order reaction with a half-...
Questions


Computers and Technology, 21.09.2021 19:10

Business, 21.09.2021 19:10



Chemistry, 21.09.2021 19:10


Chemistry, 21.09.2021 19:10

Mathematics, 21.09.2021 19:10





Mathematics, 21.09.2021 19:20

Mathematics, 21.09.2021 19:20

Physics, 21.09.2021 19:20


Mathematics, 21.09.2021 19:20

Business, 21.09.2021 19:20