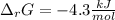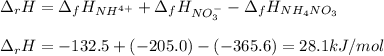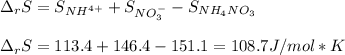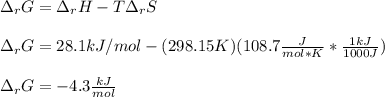Chemistry, 12.08.2020 05:01 apolloplays10
Consider the following chemical equation: NH4NO3(s)⟶NH+4(aq)+NO−3(aq) What is the standard change in free energy in kJmol at 298.15K? The heat of formation data are as follows: ΔH∘f, NH4NO3(s)=-365.6kJmolΔH∘f, NH+4(aq)=-132.5kJmolΔH∘f, NO−3(aq)=-205.0kJmol The standard entropy data are as follows: S∘NH4NO3(s)=151.1Jmol KS∘NH+4(aq)=113.4Jmol KS∘NO−3(aq)=146.4Jmol K Your answer should include two significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

You know the right answer?
Consider the following chemical equation: NH4NO3(s)⟶NH+4(aq)+NO−3(aq) What is the standard change in...
Questions


History, 17.02.2021 22:20


Mathematics, 17.02.2021 22:20


Social Studies, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20


History, 17.02.2021 22:20



History, 17.02.2021 22:20


Geography, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20


Mathematics, 17.02.2021 22:20