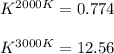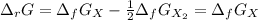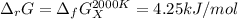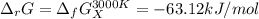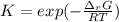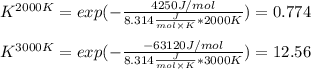Chemistry, 12.08.2020 04:01 anitadefrances
The equation represents the decomposition of a generic diatomic element in its standard state. 12X2(g)⟶X(g) Assume that the standard molar Gibbs energy of formation of X(g) is 4.25 kJ·mol−1 at 2000. K and −63.12 kJ·mol−1 at 3000. K. Determine the value of K (the thermodynamic equilibrium constant) at each temperature.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
The equation represents the decomposition of a generic diatomic element in its standard state. 12X2(...
Questions


Physics, 16.11.2020 04:50


History, 16.11.2020 04:50






English, 16.11.2020 04:50

Mathematics, 16.11.2020 04:50

Biology, 16.11.2020 04:50

History, 16.11.2020 04:50

Health, 16.11.2020 04:50

History, 16.11.2020 04:50



Mathematics, 16.11.2020 04:50


English, 16.11.2020 04:50