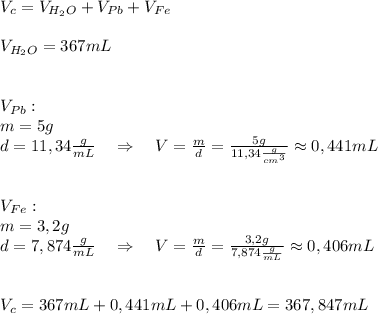A5.0 gram sample of lead and a 3.2 g sample of iron are placed into 367 ml of water. what will be the new volume level of water in units of ml? the density of lead is 11.34 g/cc and the density of iron is 7.874 g/ml. round your answer to three significant figures

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2


Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
A5.0 gram sample of lead and a 3.2 g sample of iron are placed into 367 ml of water. what will be th...
Questions




Mathematics, 21.08.2019 07:10

Mathematics, 21.08.2019 07:10

History, 21.08.2019 07:10


English, 21.08.2019 07:10


Biology, 21.08.2019 07:10

Mathematics, 21.08.2019 07:10

Mathematics, 21.08.2019 07:10


History, 21.08.2019 07:10

Mathematics, 21.08.2019 07:10


Mathematics, 21.08.2019 07:10

Mathematics, 21.08.2019 07:10

Mathematics, 21.08.2019 07:10

Mathematics, 21.08.2019 07:10