Chemistry, 12.08.2020 08:01 jothianddeepi
Ag2S + Al(s) = Al2S3 + Ag(s) (unbalanced)
1) What would the overall potential for this cell be?
2) Write the standard cell notation for an electrochemical cell consisting of an anode and cathode of the same types as in this experiment, connected through a salt bridge.

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2

Chemistry, 23.06.2019 10:00
How many grams of cupric sulfate pentahydrate are needed to prepare 50.00 ml of 0.0800m cuso4× 5h2o?
Answers: 3
You know the right answer?
Ag2S + Al(s) = Al2S3 + Ag(s) (unbalanced)
1) What would the overall potential for this cell be?
Questions

Mathematics, 13.03.2020 08:51

Mathematics, 13.03.2020 08:52

Mathematics, 13.03.2020 08:52

Mathematics, 13.03.2020 08:52


Mathematics, 13.03.2020 08:53

Mathematics, 13.03.2020 08:53


History, 13.03.2020 08:54


English, 13.03.2020 08:55

Mathematics, 13.03.2020 08:55

Mathematics, 13.03.2020 08:55







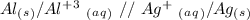
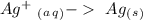
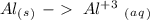
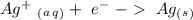 Reduction
Reduction 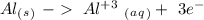 Oxidation
Oxidation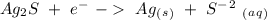 - 0.69 V
- 0.69 V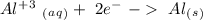 -1.66 V
-1.66 V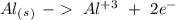 +1.66 V
+1.66 V