
Chemistry, 12.08.2020 08:01 Homepage10
Aqueous ammonia is added to a mixture of silver chloride and water. Given that Kf for the reaction between Ag+ and NH3 is large, which of the following are true?
A) The free ions are favored over the complex ion.
B) The complex ion is favored over solid silver chloride.
C) The free Ag+ ion is unstable.
D) More silver chloride will precipitate.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Aqueous ammonia is added to a mixture of silver chloride and water. Given that Kf for the reaction b...
Questions

Mathematics, 08.12.2021 20:10

Mathematics, 08.12.2021 20:10


Mathematics, 08.12.2021 20:10


Mathematics, 08.12.2021 20:10

Mathematics, 08.12.2021 20:10

English, 08.12.2021 20:10

Mathematics, 08.12.2021 20:10


History, 08.12.2021 20:10

Mathematics, 08.12.2021 20:10

Social Studies, 08.12.2021 20:10

German, 08.12.2021 20:10





Health, 08.12.2021 20:10

Mathematics, 08.12.2021 20:10

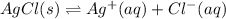
![Ag^+(aq)+2NH_3(aq)\rightleftharpoons [Ag(NH_3)_2]^+(aq)](/tpl/images/0720/0283/481b4.png)


