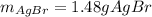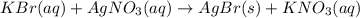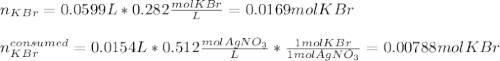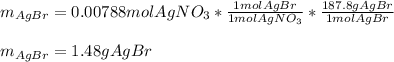Chemistry, 14.08.2020 01:01 jakobrobinette
g A chemist combines 59.9 mL of 0.282 M potassium bromide with 15.4 mL of 0.512 M silver nitrate. (a) How many grams of silver bromide will precipitate

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
g A chemist combines 59.9 mL of 0.282 M potassium bromide with 15.4 mL of 0.512 M silver nitrate. (a...
Questions

History, 21.02.2021 21:30

Mathematics, 21.02.2021 21:30


English, 21.02.2021 21:30


Mathematics, 21.02.2021 21:30


History, 21.02.2021 21:30

Spanish, 21.02.2021 21:30



Chemistry, 21.02.2021 21:30


Mathematics, 21.02.2021 21:30

Mathematics, 21.02.2021 21:30

History, 21.02.2021 21:30

Physics, 21.02.2021 21:30

Mathematics, 21.02.2021 21:30