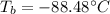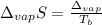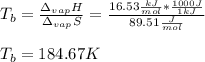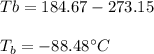Chemistry, 14.08.2020 01:01 jacobdesalvo9890
The ΔHvap of nitrous oxide is 16.53 kJ · mol−1 and its ΔSvap is 89.51 J · mol−1 · K−1. What it the boiling point of nitrous oxide?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
The ΔHvap of nitrous oxide is 16.53 kJ · mol−1 and its ΔSvap is 89.51 J · mol−1 · K−1. What it the b...
Questions

Mathematics, 26.09.2021 01:40

Mathematics, 26.09.2021 01:40



Biology, 26.09.2021 01:40

English, 26.09.2021 01:40

Mathematics, 26.09.2021 01:40

Mathematics, 26.09.2021 01:40


Mathematics, 26.09.2021 01:50

Mathematics, 26.09.2021 01:50

English, 26.09.2021 01:50

English, 26.09.2021 01:50


Mathematics, 26.09.2021 01:50


Mathematics, 26.09.2021 01:50

Mathematics, 26.09.2021 01:50