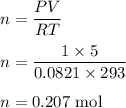Chemistry, 14.08.2020 01:01 rodriguezscarlet1713
PV = nRT. If P = 1 atm, V = 5.0 liter, R = 0.0821 L. atm/mol. K, and T = 293 K; what is the value of n?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2


Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1
You know the right answer?
PV = nRT. If P = 1 atm, V = 5.0 liter, R = 0.0821 L. atm/mol. K, and T = 293 K; what is the value of...
Questions

Mathematics, 26.11.2021 02:40





History, 26.11.2021 02:40



Mathematics, 26.11.2021 02:40

Social Studies, 26.11.2021 02:40

History, 26.11.2021 02:40


Biology, 26.11.2021 02:40

Business, 26.11.2021 02:50


Social Studies, 26.11.2021 02:50




Business, 26.11.2021 02:50